| Molecular Structure Calculations |
- Chemistry Lewis Dot Structure Calculator Worksheet
- Chemistry Lewis Dot Structure Calculator Online
- Lewis Dot Chemistry
- Lewis Dot Structure Chemistry
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. How the molecule might react with other molecules. The physical properties of the molecule (like boiling point, surface tension, etc.).
- Follow these simple steps to correctly draw a Lewis dot structure: Add up the total number of valence electrons found in the entire compound. Don’t forget to include any positive or negative charges when determining this. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only.
- As of 07/12/05 there are 1056 structures in the database. A Best Lewis Structure and Donor Acceptor Interactions Tutorial is available to help you interpret those output sections. These Lewis structure calculations are done using NBO Analysis. Answer some Study Questions to help your understanding of some interesting chemistry.
Colby Chemistry, Paul J. Schupf Computational Chemistry Lab
The simple theories of bonding that we learn in General Chemistry are powerfuland useful. These theories, which include Lewis structures, VSEPR, andhybridization, are simple models that help predict chemicalproperties. However, Lewis dot structures and hybridization are approximationsthat may or may not match reality. We should verify the usefulness of oursimple predictions with molecular orbital theory. If thetheoretical calculations are done carefully, we can learn a lot about chemical structure by comparing our Lewis structures and hybridizationarguments with the molecular orbitals.The calculations in this database includebond lengths, angles, atomic charges, the dipole moment,bond orders, and molecular orbital energies. The best Lewis structure thatfits the molecular orbitals is also calculated, so you can directlycompare with your predictions. This best Lewis structure is presented withformal electron pair localized bonds and the hybridization of the atomicorbitals used to form these localized bonds. The Chime plugin is neededto see the 3-D structure of the molecules in these pages. See thelink at the bottom of the page for the Chime plugin.
Molecular orbital theory is based on approximations also. These calculationsare done with some of the best available calculation methods (DFT forgeometry and molecular orbital energies and ab initio for properties).We use Alain St-Amant's DeFT program (University of Ottawa).
The Molecular Structure Input Form, see below, will allow you to do calculationsfor molecules not in the database.These calculations take time; 1-2 hours in some cases.
You can use the Formula Search page or browse the links below. As of 07/12/05 there are 1056structures in the database.
A Best Lewis Structure and Donor Acceptor Interactions Tutorialis available to help you interpret those output sections. These Lewisstructure calculations are done using NBO Analysis.
Answer some Study Questions to help your understanding of some interesting chemistry.
Example Molecular Orbital Results
LiHLiFLiClLiOHLiCNLiBrC2N2NOO2COF2Many More Diatomic molecules and ions
H3+Li2OBeH2BeCl2diboraneBH3BH2CNBH2SH
BF3BF32-BF4+BF2O-BCl3BH3NH3BH3COBH3PH3BO2NO
C3C5H2OH3O+H4O+O3O4CO2OCScyclic CO2CO2-HCO+HOC+
N3 radicalN3 quartetHN3N3-NCOHNCNHCNNO
NO2NO2+NO2-HOONNOO-NO2- triplet NO22+NO3-NO3-triplet NO2O-
N2Ocyclic N2Ocyclic N2O2N2O4NO2NO
ONOOHN2H2N2H2 tripletH2NNH2NN tripletNH2FNHF2NF3NF4-N2F2N2F4BNHF
HNCl+NH2ClN3ClNCl2NCl3NOClONClNClOClNO2ClNOOt-ClONOOCl2
OF2FOO•FOOFFNO2FOONF3-Cl3-Cl3FClF2+
AlH3AlF3AlCl3Al2Cl6
SiH4SiH3SiH3SiO2
P2PCl3SO2SO3SO3-SOCl2SO2Cl2ClO2ClOOClO2+ClO2-ClOO-
FClOFClO2K2O
Many More Binary Hydrides and their Anions, Cations, and Radicals
Many More Triatomic molecules and their Anions, Cations, and Radicals
Many More Period 3 Compounds, Al, Si, P, S, and Cl
Many More Period 4 Compounds, Ga, Ge, As, Se, and Br
Acids
Onium Ions:NH 4+NH3F+NH3Cl+H3O+H3O2+H2F+PH4+H3S+H2Cl+H2CN+
4+NH3F+NH3Cl+H3O+H3O2+H2F+PH4+H3S+H2Cl+H2CN+Hydrides:HFHClHCNHCN tripletHNCHNCOHOCNHONCHCNOHNCSHSCNHN3
H2N2H2N=NN2H4H2O2P2H4H2SH2
:max_bytes(150000):strip_icc()/Lewis-dot-58f78f405f9b581d5938e617.jpg) S2
S2Oxyacids:H2CO3HONHNOHNO2HNO3H2O2
Chemistry Lewis Dot Structure Calculator Worksheet
HOFHOClHClO3HClO4H3PO2H3PO3H
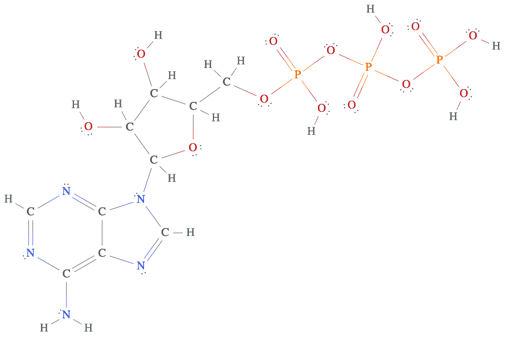 3PO4HSOHH2SO3H2SO4
3PO4HSOHH2SO3H2SO4Anions
Hydride Conjugates:F-Cl-OH-CN-NCO-CNO-NCS-CNS-NSC-N3-HN2-N2H3-HOO-P2H3-HS-
Oxyanions:HCO3-CO32-CO3OH-CO3O2-NO-NO2-NO3-HO2-O22-OF-OCl-ClO2-
ClO3-ClO4-H2PO2-H2PO3-H2PO4-HPO42-PO43-HOS-HSO-
HSO3-SO32-HSO4-SO42-S2O32-
Many MoreHydroxyl Compounds, Donor-Acceptor Oxides, Oxyacids, and Anions
Many MoreFormally Double Bonded Hydroxyl Compounds, Donor-Acceptor Oxides, and Oxyacids (e.g.Carbonic and Nitric acid)
Organics
ethaneethyleneacetyleneH2C=C, vinylidenemethanolformaldehydeformic acidmethylamineCF3Cl
Many More Carbon Compounds, Organics, and Organic Reagents
Many MoreOrganic Radical Cations, Neutral Radicals, Cations, and Anions
Oxides
NH3->OCH3NH2->OCH2NH->OCH3OH->OCH2O->OCH2O->O tripletH2N2->OPH3->OCH3PH2->OH2S->OCH3SH->OCH3F->OCH3OFCH3Cl->OH2S->O2
Reactive Intermediates
OH radicalHOO radicalH2O2+ radicalHCO3 radicalCO3- radical CO3OH-CO3O2-peroxodicarbonate dianion, O2COOCO22-methylene singlet (CH2)methylene triplet (CH2)methyl radical (CH3)CH3+
ethyl radical (CH3CH2)CH3CH2+ethylene tripletcyclopropane radicalHCC•HCC-
CH3NH-CH3OH+CH3OH2+CH3O-CH3O radicalCH2OH+CH3CO+CH2CHO-
CF2 singletCF2 tripletCF3•CF3+CCl2 singletCCl2 triplet
CHCl singletCHCl tripletCHBr singletCHBr triplet
H2CF+CH2Cl+CH2Cl-trans-C2H4Cl2+Cl2C=C singlet
allyl+allyl radicalallyl-allylalcoholradicalallylchloride radical1-chloropropane radical
HCONH-N3 radicalN33+ singlet N33+ triplet N2H+
Hydrogen Bonded and Neutral Complexes
H2O dimerNH3 dimerHF...waterHF...NH3HCl...NH3H2O...H2SH2S...H2OSO2...H2OH2O...formaldehydeHCN...formaldehydeHCN...H2CCwater...COwater...HPO2CO2...H2CO2
Chemistry Lewis Dot Structure Calculator Online
...CO2CO2...waterH2O...SO3H2S...SO3PCl3...Cl2Cl2...Cl2CH3radical...H2
 H2O...Cl•
H2O...Cl•Ion-Molecule Complexes
Li+..H2OLi+..(HLewis Dot Chemistry
2O)2BeCl22+water...superoxide-Be2+...H2C=CH2 unsymmetricalBe2+...H2C=CH2 symmetricalBe+...H2C=CH2 unsymmetricalBe+...H2C=CH2 symmetrical
FHF-CO2F-H2...OH-water...HCONH-O3...Br-
H3O+...H2OH3O+...CO2H3O+...N2H3O+...HO•NO+...H2ONO+...N2NO+...O2
Atomic and Ionic Energies
atomic energiescation energiesanion energiesWeird, Wacky, High Energy Structures
HCl-Lewis Dot Structure Chemistry
H3ClCH42+COH2CH2..HClCH3OHCH+HNCO-cyclicC4N2CO2O2-CO22-BH4+AlH4+FClPClPF4+F-ClF4-Go to the
Molecular Structure Input Form
Get your
Results Here
SRC='http://www.colby.edu/chemistry/OChem/buttons.dir/chime.GIF'ALIGN='BOTTOM'>
About the calculation methods
A bond is the sharing of 2 electrons. Covalent bonds share electrons in order to form a stable octet around each atom in the molecules. Hydrogen is the exception it only requires 2 electrons (a duet) to be stable. How do we draw a covalent Lewis Dot Structure? Level 1 (basic) 1. Add up all the valance electrons of the atoms involved. ex CF4 So C has 4 and F has 7 (x4 we have 4Fs) = 32 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by F's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 8 electrons as 4 bonds. We have 32-8= 24 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 32 electrons are now in place, count the dots around each F. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 4 bonds (2electrons) for its 8. DONE Level 2 (Double and Triple bonds) Same rules apply until #4 1. Add up all the valance electrons of the atoms involved. ex CO2 So C has 4 and O has 6 (x2 ) = 16 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by O's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 4 electrons as 2 bonds. We have 16-4=12 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 16 electrons are now in place, count the dots around each O. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 2 bonds (2electrons) for its 4....? We need 8, so move a pair of electrons from the O to between the C and O. It will share 2 pairs of electrons instead of 1. It now has a double bond instead of a single bond.
now they all have an octet, it cleans up like this Make it symmetrical. Level 3-Lewis Dots of Polyatomic Ions Same rules apply, at the end they get brackets and a charge AP Chemistry and or College Level Rules 1. Determine whether the compound is covalent or ionic. If covalent, treat the entire molecule. If ionic, treat each ion separately. Compounds of low electronegativity metals with high electronegativity nonmetals (DEN > 1.7) are ionic as are compounds of metals with polyatomic anions. For a monoatomic ion, the electronic configuration of the ion represents the correct Lewis structure. For compounds containing complex ions, you must learn to recognize the formulas of cations and anions. 2. Determine the total number of valence electrons available to the molecule or ion by:
3. Organize the atoms so there is a central atom (usually the least electronegative) surrounded by ligand (outer) atoms. Hydrogen is never the central atom. 4. Determine a provisional electron distribution by arranging the electron pairs (E.P.) in the following manner until all available pairs have been distributed:
5. Calculate the formal charge (F) on the central atom.
6. If the central atom formal charge is zero or is equal to the charge on the species, the provisional electron distribution from (4) is correct. Calculate the formal charge of the ligand atoms to complete the Lewis structure. 7. If the structure is not correct, calculate the formal charge on each of the ligand atoms. Then to obtain the correct structure, form a multiple bond by sharing an electron pair from the ligand atom that has the most negative formal charge.
8. Recalculate the formal charge of each atom to complete the Lewis structure. on to Formal Charge Chemical Demonstration Videos |